Biological Validation of Continuous Flow Effluent Decontamination Systems


As the users of continuous flow biowaste inactivation systems continue to study higher levels of hazardous microorganisms in pharma and biologics, there is an increased need for effective, routine biological validation. Current validation methodologies for continuous systems are either labor intensive, costly, or are prone to delivering inconsistent or inaccurate results.
Continuous flow systems work by raising the temperature of the effluent in heat exchangers as it continuously moves through the system piping. The waste heats up quickly in the heat exchanger and is held at the treatment temperature by flowing through a long length of tube, before continuing to a cooling heat exchanger before discharge.
In a continuous flow system, there are pumps, heat exchangers, valves and instruments. This makes for an extreme flow path for either a spore strip or a glass ampule. Bioindicators simply cannot make it through the system. Additionally, the system remains under pressure at high temperature, making exposure to contaminated biowaste a concern for validation personnel.
How do we solve these problems and validate the system?
What We’re Already Familiar With:

Traditional Thermal Batch EDS Biowell
Batch Effluent Decontamination Systems: With a batch EDS, specially-designed biowells can be located in the treatment tank. The spore strips can be placed in the biowell, and the system can process a cycle as normal. This works well because the bioindicators can be subjected to normal treatment cycle conditions without damaging them, is easily implemented regularly, low cost, and delivers accurate results.
Autoclaves: Most users of continuous flow biowaste inactivation systems are familiar with validation of autoclaves. Due to the way autoclaves work, Self-Contained Biological Indicators (SCBIs) or spore strips can be placed throughout a load and retrieved upon completion of a cycle. This works well because the bioindicators can easily be subjected to the autoclave’s cycle without destroying them.
Current Attempts:

SCBI in Holder
SCBI in Holder: Other vendors have attempted to validate using a SCBI inside of a sanitary fitting holder. At first glance, this method appears to be acceptable. The bioindicators can be exposed directly to the process and they are relatively low cost. But problems arise with the implementation. Due to the pressurization and constant flow of contaminated biowaste, there are a variety of bypass valves, depressurization valves and drain valves which must be configured.
Remember, the total treatment time for a continuous flow effluent decontamination system ranges only from 60 to 120 seconds in most cases. The length of time required to cool down, depressurize and extract the bioindicator means the bioindicator will be exposed to likely double or triple the prescribed exposure time. This does not mimic the actual treatment time and results will be skewed.
Full Scale Spore Suspensions:
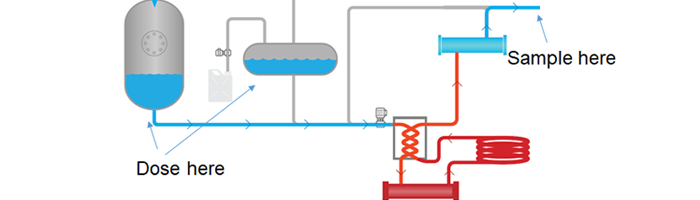
Full-Scale Spore Suspension
One method for validating a continuous flow treatment system is to spike the initial buffer tank or startup tank with a spore suspension. In this process, users would purchase a large quantity of spores, dose the buffer tank with an appropriate amount to mimic the process, and take a sampling at the end of the process to test. This method works well, and can achieve accurate validation. However, there are major drawbacks to this method.
In order to ensure that the sampling comes from the spore suspension, users will need to “clean” the buffer tank or startup tank, then add the proper dose of spores to assure a viable test. Due to the large quantities of spores required to run this test, cost becomes an issue. For Geobacillus Stearothermophilus Suspensions, it costs approximately $500 US for each liter with 106 Population, or about $19,000 US per 10 gallons (38 Liters). Due to the high costs, this method is not done with regularity, and if used at all, is rare.
PRI Bio’s Three-Pronged Validation Approach – We’ve Made it Easier:
PRI Bio has developed a three-pronged approach to achieving cost-effective, low labor, accurate validation that can be done on a routine basis.
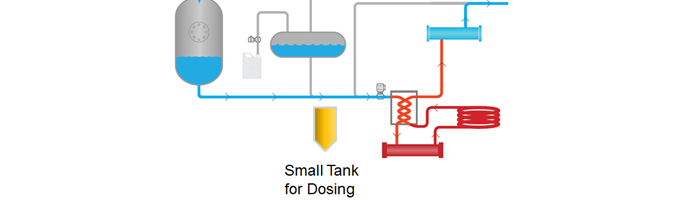
Illustration of PRI Dosing Tank
1) New Spore Suspension Protocol: we have developed a spore suspension protocol that reduces the costs, volume of spores, and labor associated with it. We can attach a specifically designed spore tank to greatly minimize the volume required for testing. Since we bypass the buffer and startup tanks, we reduce the amount of cleaning required, and allow for hot switch-over, allowing validation to take place at any time.
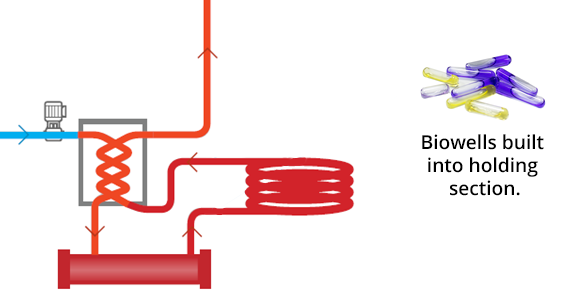
PRI Biowells Build Into Holding Section
2) Specially Designed Biowell: We have designed a new biowell for the continuous flow treatment systems, which enable the routine use of SCBI’s in similar fashion as the traditional batch systems. The biowell is built directly into the treatment piping. Since the biowell is not directly exposed to process liquid, there is no risk of exposure to personnel. This offers a low cost, low labor method that can be done at any time, without having to interrupt the process.
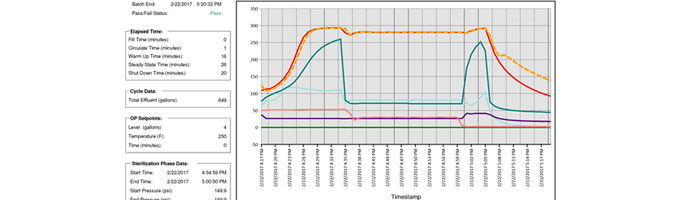
PRI Batch Report
3) Proof of Process Software: In addition to the routine validation protocols, users can gain ongoing assurance of system operation with an electronic data monitoring system. PRI Bio has developed an accurate electronic batch reporting system that logs all input and critical process parameters, trending data over a designated time period. The system displays a constant log of holding coil inlet and outlet temperature, buffer tank pressure, loop flow rate, pump discharge pressure, and drain temperature among others. With all these data points in place users can check the display or data report at any time and deliver an electronic report and archive data for future use.
With this three-pronged approach to the validation of continuous biowaste inactivation systems operators have multiple options for validation, from minimized cost of spore suspension testing, user-friendly SCBI testing with biowell, and comprehensive data logging and reporting. While validation of continuous flow biowaste inactivation systems presents significant hurdles, it can be achieved using these new methods.
For more information about validation, and to get a quote on an AutoFlow Continuous Flow biowaste treatment system, contact us here.
Categories & Tags
ISO 9001:2015
