AutoFlow™Continuous Liquid Biokill System
Continuous flow EDS suitable for pharmaceutical applications such as biologics, virology, vaccine, GMO and CMO pilot plant research labs and large-scale continuous manufacturing facilities. Capable of efficiently treating both small and large volumes of liquid process waste.

Efficient Biowaste Inactivation

Available for small or large volumes.
The AutoFlow™ is a liquid biowaste inactivation system designed for multi-purpose pharmaceutical processing and production facilities with biowaste effluent, with low solids content – perfect for cell cultures.
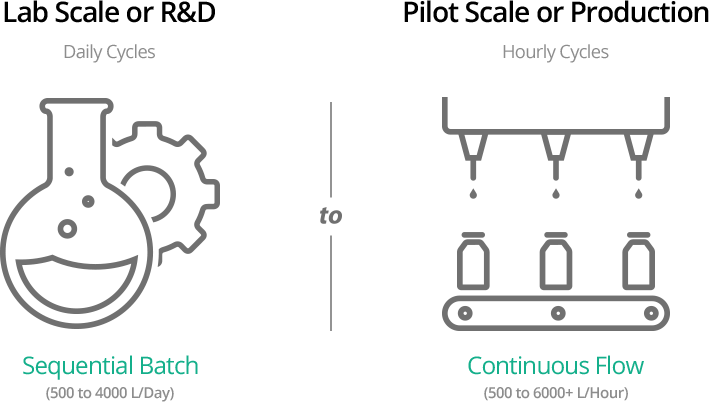
- continuous liquid biokill- 500 to 6,000+ L/Hour
- sequential batch liquid biokill – 500 to 4,000 L/Day
- high efficiency – low install, low operating costs
- fully automatic – easy to use, continuous operation
- compact design – modular, flexible, energy efficient
- hygienic construction – manufactured for pharma environments
- safe, reliable operation – self-cleaning CIP, sterile filter options

Biological Validation By Design

The AutoFlow™ effluent decontamination system includes design features to allow routine validation of the system. We understand the importance of a properly functioning biowaste treatment system, and that our users require routine validation. It is for these reasons that all of our biowaste treatment systems have built-in validation design features to allow staff to ensure the process is effective and leads to consistent results, without making the process cumbersome or inconvenient for you.

Compact Footprint, Fully Automated

Easy to use, while optimizing facility space.
The AutoFlow™ liquid inactivation system is designed for the safety of laboratory staff. It is fully automated, and once operation has been started, does not require any user contact with the system.
- effluent sterilization with adjustable temperature up to 150 degrees C
- PLC-controlled biowaste treatment and cleaning
- simple connections allow for unit mobility as needed
- constructed of lab-safe materials
- low energy consumption
- environmentally friendly

Built for Safety & Control

Clean-in-Place, Automatic Mode, Data Recording
The advanced PLC control module provides automatic operation and monitoring of the entire system, including data logs, alarms, and sensor information. The touchscreen control panel can be mounted directly to the unit, or remotely, depending upon client needs.
- visual graphic representation of operation
- simple automatic mode – runs continuously
- chemical clean-in-place to maintain full flow
- steam sterilization of individual components
- leak detection system: alarms and visual controls
- alarms for valves, sensors, temperature, pressure, and liquid levels
- recording and trending of treatment data
- password-protected operating parameter, set-point control
- fail safe system shutdown in case of energy failure
- optional redundant pumps

High Quality Construction

316L SS, Orbitally Welded, Sanitary Fittings
Each of our AutoFlow™ liquid waste decontamination systems are designed for pharmaceutical environments, and are constructed out of high grade 316L stainless steel. Systems are welded using an orbital welder in a dedicated space to maintain cleanliness.
Additionally, each liquid biowaste inactivation system uses sanitary gasketed tri-clamp fittings, to prevent exposed threaded connections.

Standard Sizes & Specifications
| Model | Capacity (Daily or Hourly) | BSL | Heat Type |
| AFs-500 | 500 LPD (132 Gal) | 1, 2, 3 | Electric |
| AFs-1000 | 1000 LPD (264 Gal) | 1, 2, 3 | Electric |
| AFs-2000 | 2000 LPD (528 Gal) | 1, 2, 3 | Electric or Steam |
| AFs-4000 | 4000 LPD (1057 Gal) | 1, 2, 3 | Electric or Steam |
| AFc-500 | 500 LPH (132 Gal) | 1, 2, 3 | Electric or Steam |
| AFc-1000 | 1000 LPH (264 Gal) | 1, 2, 3 | Electric or Steam |
| AFc-2000 | 2000 LPH (528 Gal) | 1, 2, 3 | Steam |
| AFc-4000 | 4000 LPH (1057 Gal) | 1, 2, 3 | Steam |
| AFc-6000 | 6000 LPH (1585 Gal) | 1, 2, 3 | Steam |