Determine Your Biosafety Level Design Requirements

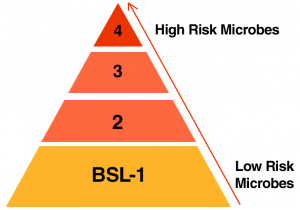
Biosafety Level 1 represents a basic level of containment that relies on standard microbiological practices with no special primary or secondary barriers recommended, other than a sink for hand washing.
Biosafety Level 2 practices, equipment, and facility design and construction are applicable to clinical, diagnostic, teaching, and other laboratories in which work is done with the broad spectrum of indigenous moderate-risk agents that are present in the community and associated with human disease of varying severity. Secondary barriers, such as hand washing sinks and waste decontamination facilities, must be available to reduce potential environmental contamination.

Biosafety Level 3 practices, safety equipment, and facility design and construction are applicable to clinical, diagnostic, teaching, research, or production facilities in which work is done with indigenous or exotic agents with a potential for respiratory transmission, and which may cause serious and potentially lethal infection. Secondary barriers for this level include controlled access to the laboratory and ventilation requirements that minimize the release of infectious aerosols from the laboratory.
Biosafety Level 4 practices, safety equipment, and facility design and construction are applicable for work with dangerous and exotic agents that pose a high individual risk of life-threatening disease, which may be transmitted via the aerosol route and for which there is no available vaccine or therapy. The primary hazards to personnel working with BSL-4 agents are respiratory exposure to infectious aerosols, mucous membrane or broken skin exposure to infectious droplets, and autoinoculation. All manipulations of potentially infectious diagnostic materials, isolates, and naturally or experimentally infected animals, pose a high risk of exposure and infection to laboratory personnel, the community, and the environment. The laboratory worker’s complete isolation from aerosolized infectious materials is accomplished primarily by working in a Class III BSC or in a full-body, air-supplied positive-pressure personnel suit. The BSL-4 facility itself is generally a separate building or completely isolated zone with complex, specialized ventilation requirements and waste management systems to prevent release of viable agents to the environment.
As is depicted above, the microorganisms studied at each BSL carry their own unique properties for treatment. This has resulted in varying levels of disinfection and treatment with prescribed requirements for achieving a particular level. Learn more about these sterilization levels and the Effluent Decontamination Systems that can achieve each level here.
PRI Bio’s Effluent Decontamination Systems take these factors into account, and are built with special considerations for containment. To summarize, these can include sterile filtration, advanced pressure relief systems, isolated valve control, individual air shut off valves for each control device, batch process data reporting, and more.
Effluent Decontamination Systems built by PRI are designed to safely handle emergency situations. In the unlikely event that the equipment should lose power, air, steam, or other catastrophic failures the components will revert to a fail-safe mode. Depending on each function or component of the system, they may move into either a fail-close, fail-open, or trigger a backup response (i.e.: sensors, valves, and controllers).
Some facilities are built with different BSL lab suites. Our systems can be design at a BSL-3 level to accept high containment waste, while also functioning as the treatment system for the BSL-2 suite. PRI Bio has experience at each Bio-Safety Level, and can provide a customized solution for your unique requirements.
Categories & Tags
ISO 9001:2015
